Shop
Why Do Guinness Bubbles Sink?
Scientific research with all the right priorities.
Guinness is a strange beer. Its color is a dark maroon but its taste is light and creamy. It’s a stout, but at 4.2% ABV, it’s got less bite than Budweiser. And, most curiously, as you wait for it to settle, the bubbles appear to be sinking, rather than floating to the top.
Actually, there’s been a disturbing amount of scientific research devoted to understanding this last point. Unsurprisingly, much of this work was done out of Ireland. Dr. William Lee, a former Professor of Applied Mathematics at the University of Limerick, determined that this phenomenon is due to two important chemical and physical properties: nitrogen gas and the shape of a beer glass.
First, the gas. Guinness is among the few beers to which brewmasters synthetically add nitrogen gas during the brewing process. Nitrogen, which you’re breathing right now, is the source of many of the weird properties of Guinness. First, nitrogen is a rather inert (non-reactive) gas in water, whereas in more traditional beers, carbon dioxide likes to react with water and generate free hydrogen ions, making your beer more acidic. This helps create that smooth, creamy taste. Second, relative to carbon dioxide bubbles, nitrogen bubbles are tiny, having roughly the diameter of a human hair.
A nerdy aside: The nitrogen is forced out of the beer and into gaseous form (bubbles) by the “restrictor disc” that the beer is forced through when it’s poured from a tap. Check the tap next time you’re at a bar: the “stout faucet,” sometimes called “European Specialty Faucet,” is different than the one used to pour Coors Light. In bottle or can form, the bubbles are incited by that little plastic widget that rattles around after you finish your beer.

Photography by Marion Wacker/Flickr
Now, imagine for a second that you’re in a pool holding both a tennis ball, which will represent a bubble of nitrogen, and a basketball, which will represent a bubble of carbon dioxide.
So the beer’s been poured and we’ve established that the bubbles are tiny. Now, imagine for a second that you’re in a pool holding both a tennis ball, which will represent a bubble of nitrogen, and a basketball, which will represent a bubble of carbon dioxide. It’s a lot easier to hold that tennis ball underwater when compared to the basketball, because there’s a smaller buoyant force trying to push the tennis ball up. This is the first critical piece of information to understanding why bubbles in Guinness sink: tiny nitrogen bubbles have a very small buoyant force acting upon them, so it’s not very difficult to pull them down.
So what is pulling these bubbles down? Dr. William Lee and collaborators published a series of papers in 2011 and 2013 to find out. Lee carried out a very simple experiment: he poured Guinness into a normal pint glass, which narrows near its base, and he poured Guinness into an anti-pint glass, which widens near its base. In the normal pint glass, he witnessed the bubbles on the sides of the glass sink to the bottom, but in the anti-pint glass, bubbles near the sides of the glass began to rise. Dr. Lee determined that the shape of a normal pint glass creates areas of low bubble density along the edges of the glass, and areas of high bubble density in the middle of the glass.
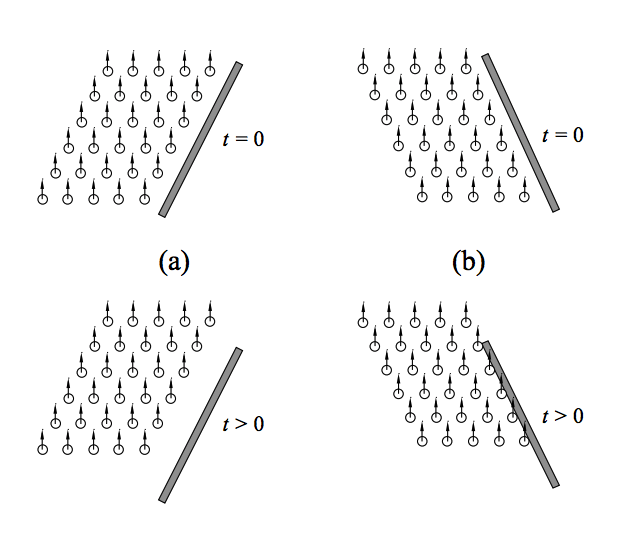
Image Credit: Why do bubbles in Guinness sink?
What does this mean? To illustrate this point, let’s take a look at one of the figures from Dr. Lee’s 2013 paper. In figure (a), we have a normal pint glass. As bubbles start to float to the top (bottom figure), a gap forms between the bubbles and the sides of the glass, which creates an area of low bubble density. In figure (b), we have an anti-pint glass which gets wider near the bottom of the glass. Here, as bubbles begin to float (bottom figure), they hit the sides of the glass and create an area of high bubble density.
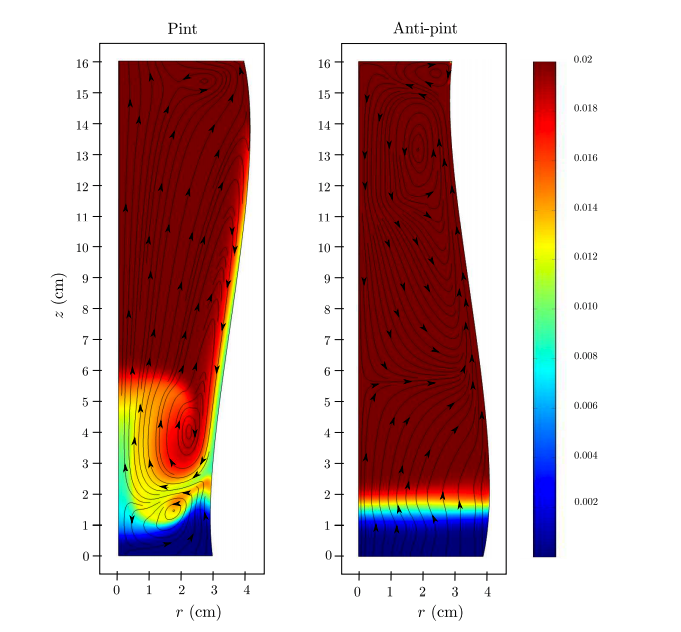
Image Credit: Why do bubbles in Guinness sink?
This is the second critical piece of information to understanding why Guinness bubbles sink: In a normal pint glass, in which the bubbles pull away from the sides of the glass and cluster in the center, there’s a column of very high bubble density in the center of the glass. These bubbles float upward with some (relative) force. Bubbles from the top are then pulled back down around the column in order to fill the low bubble density areas along the sides and bottom.
Think of this as watching Titanic in reverse. In this case, the Titanic is the column of nitrogen bubbles moving upward. As the Titanic sinks into the chilly North Atlantic, it created suction behind it. This is what pulls Jack under water (Rose had on a life vest). In our case, nitrogen bubbles, which travel along the sides of the glass, are pulled to the bottom of the glass along the sides of the glass. In the figure, the sinking bubbles are found in the yellow and green areas.
Think of the Titanic sinking.
Now you may be wondering: “Why the hell is any of this important?” And you would be asking a very reasonable question. But in his paper, Dr. Lee leaves the reader with a fairly interesting question, “Is the shape of the Guinness pint glass the most efficient possible, or could the settling time [AKA the time you have to wait before you drink the beer after pouring] be significantly reduced by some other, possibly non-axisymmetric, shape of pint glass?” So the real question is: How can we drink Guinness faster?
Dylan Eiger is graduate student at Duke University who is pursuing his MD/PhD. He also drinks beer occasionally.



